Last week at the Folk School, a student asked about RapidRise yeast—what is it? This is one of those questions I’ve always meant to look up again, having not previously found a good answer. I knew that RapidRise is a brand name, but what type of yeast is it actually?
Yeast Review

Good old active dry yeast
You may remember that active dry yeast is a dried-out form of yeast that is supposed to be activated (brought out of dormancy) with warm water before use. Instant yeast is also dried out, but there is no need to activate it, hence the name. With SAF instant yeast, it is the rapid drying process that enables it to be used instantly and produces its tubular shape. I learned this in a talk by someone from LaSaffre and wrote about it here: https://foodchemblog.com/2014/08/notes-from-the-science-of-yeast-talk/
Answer 1: RapidRise Is Instant Yeast, Almost
I found two articles by P.J. Hamel on the King Arthur website that seemed to mostly answer the question:
Which Yeast To Use: Choosing The Best Type For Any Recipe, 2016, https://blog.kingarthurflour.com/2016/01/29/yeast-use/
Baguettes – Take A Walk On The Wild (Yeast) Side, 2010, https://blog.kingarthurflour.com/2010/10/11/baguettes-take-a-walk-on-the-wild-yeast-side/

RapidRise yeast
The first article (the 2016 one) confirms that RapidRise is Fleischmann’s brand name, while Quick-Rise is Red Star’s; these fast-rising yeasts claim to work faster than active dry yeast. They include the words “instant yeast” on their packaging. The article also mentions LeSaffre’s SAF instant yeast, the favorite at King Arthur.
The article states that “SAF instant yeast is not the same as Red Star and Fleischmann’s fast-rising yeasts.” One difference is noted: Red Star Quick-Rise yeast is not supposed to be used for refrigerated or frozen doughs, while SAF instant yeast can be. Otherwise, it’s not clear what the difference is or what causes it.
Then a test is done comparing an active dry yeast, Quick-Rise yeast, and SAF instant yeast. The active dry yeast lags behind the others (but note * below; perhaps she had not activated the active dry yeast, and it hurt performance?), while the others perform about the same. Subtle differences are noted, but they seem negligible.
The second (2010) article digs deeper into the technical differences between the yeasts.

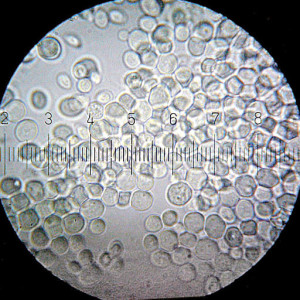
Note the round shape of active dry yeast granules
Regarding active dry yeast, the article gives a reason for activation that I have never before heard: it’s not about bringing yeast cells out of dormancy, but rather about washing away dead yeast cells to expose the live ones. It states that this activation is no longer necessary,* due to improvements in active dry yeast, which no longer has so many dead cells. (I wish I had more information about this, as it doesn’t seem to match what I heard from the yeast expert from LeSaffre.)
Regarding instant yeast, RapidRise yeast, and bread machine yeast, the article states that instant and bread machines yeasts are the same thing, but that “there’s no agreement, even among folks from the same company, as to whether RapidRise and instant yeast are the exact same yeast.”
Answer 2: There Are Genetic Differences

Note the tubular shape of RapidRise granules
I went to class all ready to discuss what I’d learned, but it turned out the student had done his own research and come up with completely difference answers.
The Bakerpedia website (which uses “quick rise dry yeast,” a.k.a. instant yeast, for the general name) says that instant yeast is a genetically engineered yeast strain that stays active after drying. There is also a special drying process that creates porous particles, while protecting yeast cells from damage. The porosity enables the particles to rehydrate rapidly but also hurts their shelf life. The yeast is also “finely ground.” References are given! But I have not had time to follow up with them. Read more here: http://bakerpedia.com/ingredients/quick-rise-dry-yeast/
This entry notes that the baker can skip some steps when using instant yeast, but remember that we like more steps because more time rising equals more flavor.
My Conclusions
It can be hard to figure out whom to trust on the Internet. My takeaway from all this is that RapidRise yeast acts basically the same as instant yeast (based on the experiment shown in the 2016 article), with the exception of cold doughs. There seems to be something going on behind the scenes (secret strains of yeast? patented drying processes?) that prevents us from learning what the specific differences are among the yeast brands.

Wait, pizza crust yeast? What the heck is that?!
Classification seems to depend on how instant yeast as a category is defined. If it simply means “yeast that starts right up without activation,” then it seems clear that RapidRise, Quick-Rise, and SAF are all instant yeasts.
If SAF somehow defines instant yeast, as the King Arthur site seems to imply, then we don’t know whether RapidRise and Quick-Rise are the same: whether they contain the same type of yeast as SAF or use the same drying process. (RapidRise yeast does have the same tubular shape as SAF.) If not, they might not be the same as SAF instant, but they’d still be in a category together; maybe we’d call it “fast-rising yeasts,” since “instant” seems to cause confusion.
Regardless, for bread making purposes, it seems that you can use any instant yeast in recipes that call for a particular brand (such as RapidRise) or in a bread machine. And, you can substitute instant yeast for active dry yeast, or vice versa, knowing that your dough might (or might not) rise a little more slowly with active dry.
If you’ve had any experiences with a certain type or brand of yeast, please share it in the comments!
 I just read a small book called Do Sourdough: Slow Bread for Busy Lives, by Andrew Whitley. [1] The book contains everything a beginner needs to know to work with sourdough, from creating a starter to using it in bread (recipes included), without any additional clutter. Mr. Whitley, a baker after my own heart, points the reader toward the cheapest solutions and the easiest methods. I have friends interested in sourdough, as a possible solution to gluten intolerance, and this is the book I will point them to.
I just read a small book called Do Sourdough: Slow Bread for Busy Lives, by Andrew Whitley. [1] The book contains everything a beginner needs to know to work with sourdough, from creating a starter to using it in bread (recipes included), without any additional clutter. Mr. Whitley, a baker after my own heart, points the reader toward the cheapest solutions and the easiest methods. I have friends interested in sourdough, as a possible solution to gluten intolerance, and this is the book I will point them to. I’ve read that, in sourdough starters, the wild yeasts are responsible for raising the dough, while the bacteria create the flavor. This never made sense to me: Don’t both kinds of microorganism perform fermentation and respiration? What’s the difference? This time, I started poking around looking for answers.
I’ve read that, in sourdough starters, the wild yeasts are responsible for raising the dough, while the bacteria create the flavor. This never made sense to me: Don’t both kinds of microorganism perform fermentation and respiration? What’s the difference? This time, I started poking around looking for answers.




 At the beginning of summer, I reviewed a book called The Science of Cheese for American Scientist Magazine. Last week, with summer winding down,
At the beginning of summer, I reviewed a book called The Science of Cheese for American Scientist Magazine. Last week, with summer winding down,